Diastereomers (sometimes called diastereoisomers) are stereoisomers that are not enantiomers.[1] Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related) stereocenters and are not mirror images of each other. When two diastereoisomers differ from each other at only one stereocenter they are epimers. Each stereocenter gives rise to two different configurations and thus to two different stereoisomers.
Diastereomers differ from enantiomers in that the latter are pairs of stereoisomers which differ in all stereocenters and are therefore mirror images of one another.[3] Enantiomers of a compound with more than one stereocenter are also diastereomers of the other stereoisomers of that compound that are not their mirror image. Diastereomers have different physical properties and different reactivity, unlike enantiomers.
Cis-trans isomerism and conformational isomerism are also forms of diastereomerism.
Diastereoselectivity is the preference for the formation of one or more than one diastereomer over the other in an organic reaction.
Example
Tartaric acid contains two asymmetric centers, but two of the "isomers" are equivalent and together are called a meso compound. This configuration is not optically active, while the remaining two isomers are D- and L- mirror images, i.e., enantiomers. The meso form is a diastereomer of the other forms.
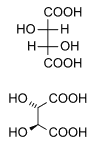 | 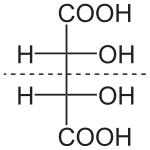 | |
| (natural) tartaric acid | D-(-)-tartaric acid | mesotartaric acid |
| (1:1) | ||
The families of 4, 5 and 6 carbon carbohydrates contain many diastereomers because of the large numbers of asymmetric centres in these molecules.

No comments:
Post a Comment